I am always thinking how to deal with the question if I am asked why using yeast to address problems in cancer. This paper (Coelho et al, 2019) published in Nature sets a good example for yeast geneticist to think how to link our work by yeast to cancer research.
Genetic instability refers to an increased frequency of genomic alterations during cell cycles, therefore, accelerates the accumulation of mutations. It is a widespread characteristic in cancer cells, which can be caused by mutations in DNA repair genes, or mutations in tumor suppressor genes. Given that diploid cells (such as human) have two alleles (one allele inherited from each parent) at each genetic locus, the mutation can occur at only a single allele (heterozygous), or at both alleles (homozygous). Usually the homozygous mutations need a second hit by LOH (loss of heterozygosity), which inactivates the other functional allele. The relative importance of these two kinds of mutations is difficult to be determined, especially in the genomes of human tumors. To overcome the difficulties, the authors took the advantage of tractable budding yeast system to assess the importance of the one- or two- hit routes to genetic instability.
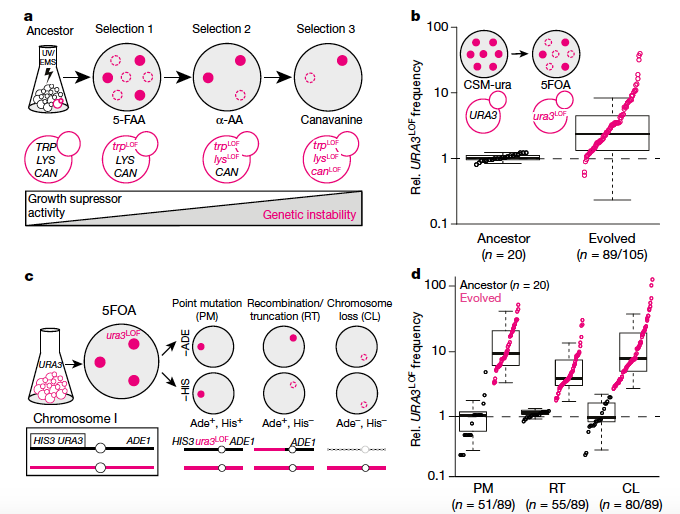
First, the authors evolved a number of yeast diploid clones for genetic instability by propagating them in three drugs that suppress cell growth. The evolved clones showed a higher rate of point mutation, mitotic recombination, and chromosome loss, indicating their genetic instability. By analyzing the segregation patterns of the spores from these clones, they found about 96% of them have one or more heterozygous mutations. Only very few have a single homozygous mutation. Next they sequenced these clones as well as the pool of their spores to identify candidate genes that correlated with genetic instability. They identified 92 genes and successfully verified a subset of them by either single copy gene deletion or engineering a single copy of the mutation into ancestral diploid, indicating that heterozygous mutation is sufficient to cause genetic instability. To further support the result, they performed another experiment to restore the ancestral version of the mutated alleles in the genetic unstable strains. They found restoring homozygosity for the wild type alleles reduced genetic instability.
As it is known, yeast shares a lot of similarities with human cells in terms of genes and metabolisms. Given that genetic instability is common for human cancer cells, it is reasonable to test the genes found in yeast that cause genetic instability into human cell lines. The authors inactivated six homologous genes in a near-haploid human cell line to monitor their corresponding genetic instability, using a gene which is known to associated with genetic instability as positive control. The tested genes showed a 4- to 440- fold increase in mutation rate compared with 150-fold increase in the positive control. Therefore, potentially these homologous genes may be linked to tumorigenesis.
This study indicates that one-hit heterozygous mutations can lead to genetic instability, which is much more often than the two-hit homozygous mutations. Although the experimental validation in human cells is limited, the authors have provided a list of candidate genes that might be important for cancer initiation and progression. This study serves as a very good example of how to use model organism (e.g. budding yeast) to explore important questions in cancer biology in a systematic and cost-effective way.